Determining density via water displacement gizmo answers – Embark on a scientific adventure with “Determining Density via Water Displacement: A Comprehensive Guide.” This exploration unveils the mysteries of density, empowering you with the knowledge to unravel the secrets of the physical world. Join us as we delve into the intricacies of this fundamental property, uncovering its applications and mastering the techniques for precise measurement.
By harnessing the power of water displacement, we embark on a journey to determine the density of various substances. Prepare to be captivated as we unravel the concepts, techniques, and applications that lie at the heart of density measurement.
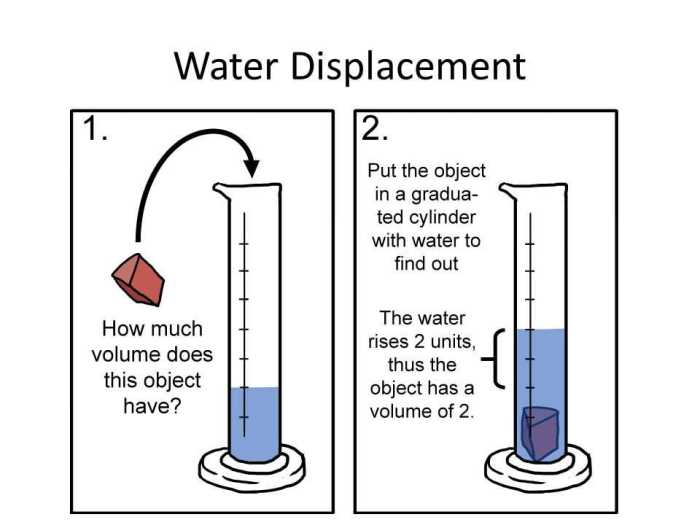
Measuring Density with Water Displacement
Density is a measure of the compactness of a substance, defined as the mass per unit volume. It is expressed in kilograms per cubic meter (kg/m 3) or grams per cubic centimeter (g/cm 3).
The water displacement method is a common technique used to measure the density of an object. It involves submerging the object in water and measuring the volume of the displaced water. The density of the object can then be calculated using the following formula:
Density = Mass of object / Volume of displaced water
Materials and Equipment
- Unknown object
- Graduated cylinder or beaker
- Water
- Scale
Procedure:
- Fill the graduated cylinder or beaker with a known volume of water.
- Record the initial volume of water.
- Submerge the object completely in the water.
- Record the final volume of water.
- Calculate the volume of displaced water by subtracting the initial volume from the final volume.
- Weigh the object on the scale.
- Calculate the density of the object using the formula provided above.
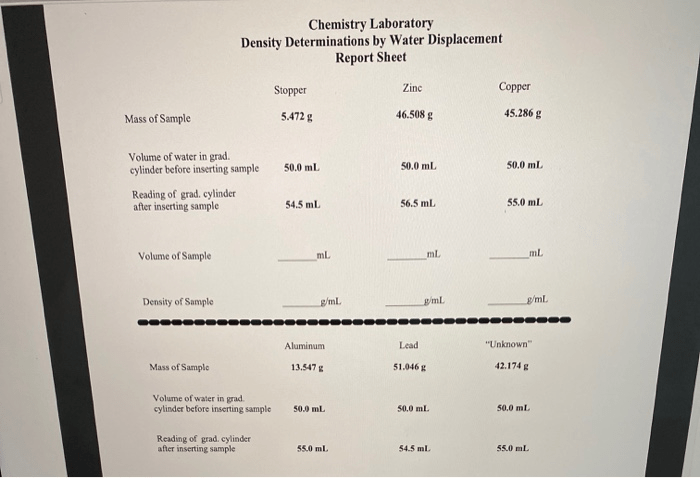
Data Collection
Create a table to organize the data collected during the experiment:
| Mass of object (g) | Volume of displaced water (cm3) | Density (g/cm3) |
|---|---|---|
Data Analysis, Determining density via water displacement gizmo answers
To analyze the data, simply divide the mass of the object by the volume of displaced water. This will give you the density of the object in g/cm 3.
Sources of Error:
- Inaccurate measurement of the volume of displaced water
- Inaccurate measurement of the mass of the object
- Air bubbles trapped on the object
To minimize errors, ensure that the graduated cylinder or beaker is calibrated and that the object is completely submerged in water. You can also repeat the experiment several times to get an average value.
Applications of Density
Density is a useful property that can be used in a variety of applications, including:
- Identifying materials:Different materials have different densities, so density can be used to identify unknown materials.
- Determining purity:The density of a substance can change if it is mixed with impurities, so density can be used to determine the purity of a substance.
- Quality control:Density can be used to ensure that products meet quality standards.
Key Questions Answered: Determining Density Via Water Displacement Gizmo Answers
What is density?
Density is a fundamental property of matter that measures the mass per unit volume of a substance. It provides insights into the compactness and heaviness of materials.
How does the water displacement method measure density?
The water displacement method involves submerging an object in water and measuring the volume of water displaced. By dividing the mass of the object by the volume of water displaced, we can determine its density.
What are the applications of density measurement?
Density measurement finds applications in various fields, including material identification, purity determination, quality control, and geological exploration.