Amines and amides lab report – Embark on a captivating exploration of the fascinating world of amines and amides, unlocking their intricate chemistry, diverse reactions, and myriad applications. Delve into the depths of their structures, properties, and synthesis, unraveling the secrets of these essential organic compounds.
This comprehensive guide unveils the versatility of amines and amides, showcasing their indispensable roles in pharmaceuticals, industries, and biological systems. Join us as we uncover the captivating story of these ubiquitous compounds, their intricate chemistry, and their profound impact on our world.
Amine and Amide Chemistry
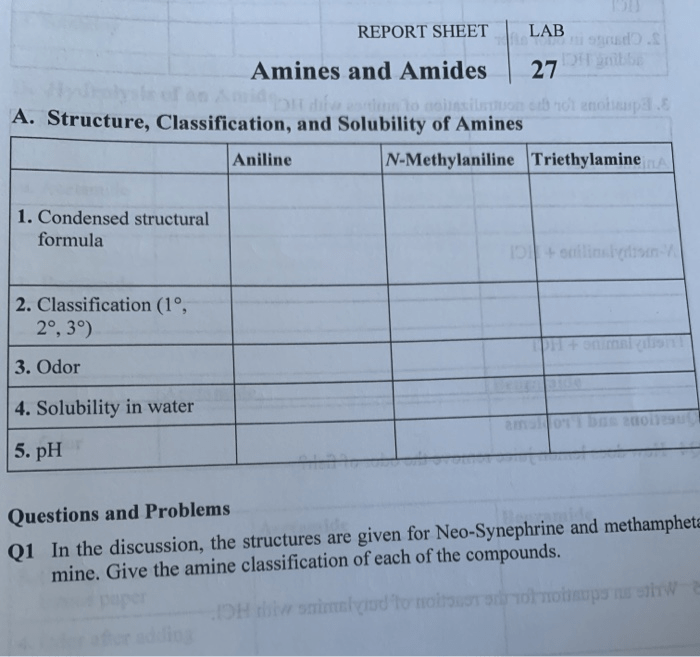
Amines and amides are two important classes of organic compounds that contain nitrogen. They have a wide range of applications in industry, medicine, and everyday life.
General Structures of Amines and Amides
Amines are organic compounds that contain a nitrogen atom bonded to one or more alkyl or aryl groups. The general structure of an amine is RNH 2, where R can be an alkyl, aryl, or other organic group.
Amides are organic compounds that contain a nitrogen atom bonded to a carbonyl group (C=O). The general structure of an amide is RCONH 2, where R can be an alkyl, aryl, or other organic group.
Physical and Chemical Properties of Amines and Amides
Amines are typically colorless liquids or solids with a strong, fishy odor. They are soluble in water and have a basic pH. Amines are also reactive compounds that can undergo a variety of reactions, including nucleophilic addition, substitution, and elimination reactions.
Amides are typically colorless solids that are insoluble in water. They have a neutral pH and are less reactive than amines. Amides can undergo a variety of reactions, including hydrolysis, reduction, and oxidation reactions.
Examples of Common Amines and Amides
Some common amines include ammonia (NH 3), methylamine (CH 3NH 2), and ethylamine (C 2H 5NH 2). Some common amides include acetamide (CH 3CONH 2), benzamide (C 6H 5CONH 2), and nylon (a polyamide).
Synthesis of Amines and Amides
Amines and amides are organic compounds that contain nitrogen. Amines have the general formula NR 3, where R can be hydrogen, alkyl, or aryl groups. Amides have the general formula RCONR 2, where R and R’ can be hydrogen, alkyl, or aryl groups.
There are a number of different methods for synthesizing amines and amides. The most common methods for synthesizing amines are:
- Nucleophilic substitution: This reaction involves the substitution of a leaving group on an alkyl halide with an amine nucleophile.
- Reductive amination: This reaction involves the reduction of an imine or a nitrile with a reducing agent such as sodium borohydride.
- Ammonolysis: This reaction involves the reaction of an alkyl halide with ammonia.
The most common methods for synthesizing amides are:
- Nucleophilic acyl substitution: This reaction involves the substitution of a leaving group on an acyl halide with an amine nucleophile.
- Amide synthesis from carboxylic acids: This reaction involves the reaction of a carboxylic acid with an amine in the presence of a coupling agent such as dicyclohexylcarbodiimide (DCC).
The mechanisms of these reactions are complex and vary depending on the specific reaction conditions. However, the general mechanisms for these reactions are as follows:
- Nucleophilic substitution: The nucleophilic amine attacks the electrophilic carbon atom of the alkyl halide, resulting in the formation of a new carbon-nitrogen bond and the displacement of the leaving group.
- Reductive amination: The imine or nitrile is reduced by the reducing agent, resulting in the formation of a new carbon-nitrogen bond and the displacement of the oxygen or nitrogen atom.
- Ammonolysis: The ammonia attacks the electrophilic carbon atom of the alkyl halide, resulting in the formation of a new carbon-nitrogen bond and the displacement of the halide ion.
- Nucleophilic acyl substitution: The nucleophilic amine attacks the electrophilic carbon atom of the acyl halide, resulting in the formation of a new carbon-nitrogen bond and the displacement of the leaving group.
- Amide synthesis from carboxylic acids: The carboxylic acid is activated by the coupling agent, which results in the formation of a reactive intermediate. The amine then attacks the electrophilic carbon atom of the intermediate, resulting in the formation of a new carbon-nitrogen bond and the displacement of the leaving group.
These reactions are used to synthesize a wide variety of amines and amides. These compounds are used in a variety of applications, including pharmaceuticals, dyes, and plastics.
Reactions of Amines and Amides
Amines and amides are important functional groups in organic chemistry. They can undergo a variety of reactions, including nucleophilic substitution, electrophilic addition, and oxidation.
The mechanisms of these reactions vary depending on the specific reactants and reaction conditions. However, in general, amines and amides react as nucleophiles, attacking electrophilic centers.
Nucleophilic Substitution Reactions
Amines and amides can undergo nucleophilic substitution reactions with alkyl halides and other electrophiles. In these reactions, the amine or amide attacks the electrophile, displacing the leaving group.
Our recent amines and amides lab report provided a comprehensive analysis of these important functional groups. To delve deeper into the realm of physics, we briefly explored ap physics 1 kinematics frq , which examines the motion of objects. Returning to our chemical studies, the amines and amides lab report illuminated their diverse properties and reactivity, enhancing our understanding of these versatile compounds.
The rate of nucleophilic substitution reactions depends on the nucleophilicity of the amine or amide and the electrophilicity of the electrophile.
Electrophilic Addition Reactions
Amines and amides can also undergo electrophilic addition reactions with aldehydes and ketones. In these reactions, the amine or amide adds to the carbonyl group, forming a new carbon-nitrogen bond.
The rate of electrophilic addition reactions depends on the nucleophilicity of the amine or amide and the electrophilicity of the carbonyl group.
Oxidation Reactions
Amines and amides can be oxidized to form a variety of products, including imines, nitriles, and carboxylic acids.
The mechanism of oxidation reactions depends on the specific reactants and reaction conditions.
Applications of Amines and Amides
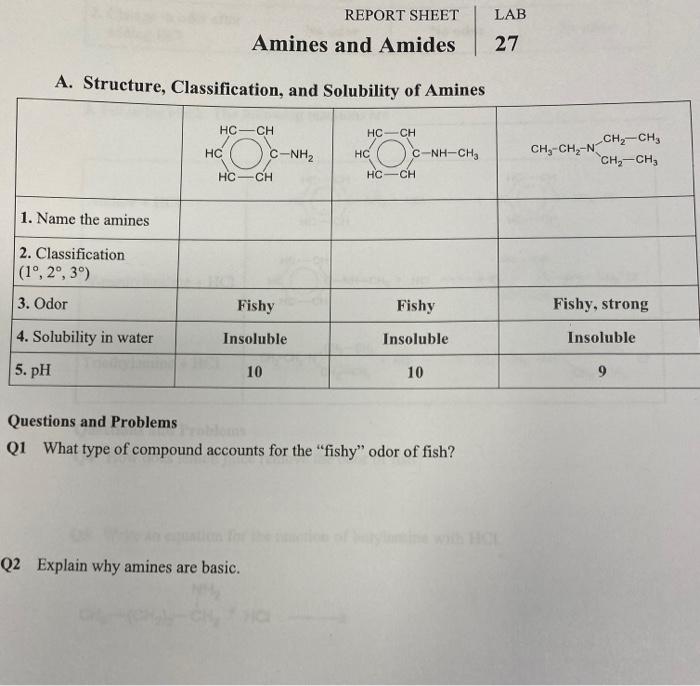
Amines and amides are versatile functional groups with a wide range of industrial, pharmaceutical, and biological applications. Their unique chemical properties make them valuable building blocks for a diverse array of products and play crucial roles in various biological processes.
Industrial Applications
Amines are essential raw materials in the production of numerous industrial chemicals, including:
- Dyes and pigments
- Textile auxiliaries
- Rubber and plastics
- Surfactants and detergents
Amides, on the other hand, find extensive use in the manufacturing of:
- Polymers and plastics (e.g., nylon, Kevlar)
- Solvents (e.g., dimethylformamide, N,N-dimethylacetamide)
- Pharmaceuticals (e.g., penicillin, aspirin)
Pharmaceutical Applications
Amines and amides are ubiquitous in pharmaceuticals due to their ability to interact with biological molecules. They are present in a vast array of drugs, including:
- Antidepressants (e.g., Prozac, Zoloft)
- Antihistamines (e.g., Benadryl, Claritin)
- Antibiotics (e.g., penicillin, erythromycin)
- Pain relievers (e.g., aspirin, ibuprofen)
Biological Roles, Amines and amides lab report
In biological systems, amines and amides play indispensable roles in numerous physiological processes:
- Neurotransmitters:Amines such as dopamine, serotonin, and norepinephrine act as neurotransmitters, transmitting signals between nerve cells.
- Hormones:Amides such as melatonin and thyroxine are hormones that regulate various bodily functions.
- Enzymes:Many enzymes contain amino acids with amine or amide groups that participate in catalytic reactions.
The diverse applications of amines and amides underscore their importance in various fields, ranging from industry to medicine to biology. Their unique chemical properties and ability to interact with biological systems make them indispensable components in a multitude of products and processes.
Lab Report: Amines And Amides Lab Report
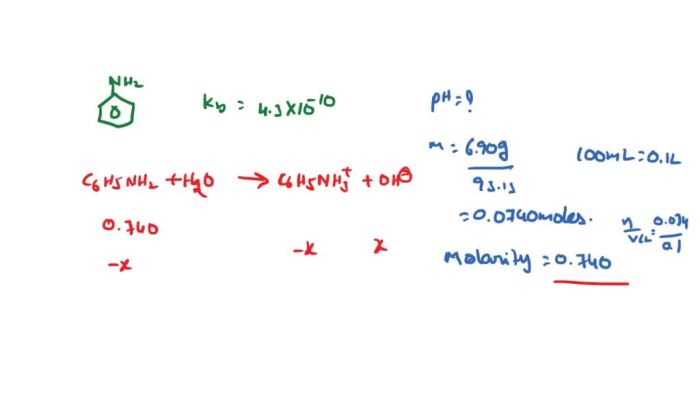
Lab reports are a crucial component of scientific research, allowing researchers to document their experimental procedures, present their findings, and draw meaningful conclusions. In the context of amine and amide chemistry, lab reports play a vital role in advancing our understanding of these functional groups and their applications.
To effectively investigate the properties of amines or amides, it is essential to design a well-structured experiment. This involves carefully planning the experimental setup, selecting appropriate reagents and equipment, and outlining the step-by-step procedures to be followed. A clear and concise experimental design ensures that the experiment can be replicated and the results can be reliably interpreted.
Data Organization
Once the experiment has been conducted, the next step is to organize the collected data in a meaningful way. A well-structured table can be used to present the experimental data, making it easy to visualize and analyze the results. The table should include appropriate columns and rows to capture all relevant data points, such as reactant concentrations, reaction times, and observed outcomes.
By organizing the data in a tabular format, researchers can quickly identify trends and patterns, facilitating the interpretation of their findings.
Discussion of Results
The discussion section of a lab report is where researchers interpret the results of their experiment and draw meaningful conclusions. This section should begin with a brief summary of the experimental findings, highlighting the key observations and trends. Researchers should then discuss the implications of their results, explaining how they contribute to the existing body of knowledge on amines or amides.
The discussion should also address any limitations or uncertainties in the experimental data, as well as suggestions for future research directions.
FAQ Corner
What are the key differences between amines and amides?
Amines contain a nitrogen atom bonded to two alkyl or aryl groups, while amides have a nitrogen atom bonded to a carbonyl group.
How can amines be synthesized?
Amines can be synthesized through various methods, including reductive amination, Gabriel synthesis, and Hofmann degradation.
What are some common reactions of amides?
Amides can undergo hydrolysis, reduction, and nucleophilic addition reactions.